Nutrient Exposure Alters Microbial Mat Composition, Structure, and Mercury Methylation in a Contaminated Watershed
Monitoring the entire periphyton microbiome, in addition to its mercury-methylating members, may be important in evaluating mercury dynamics in natural environments.
March 19, 2021
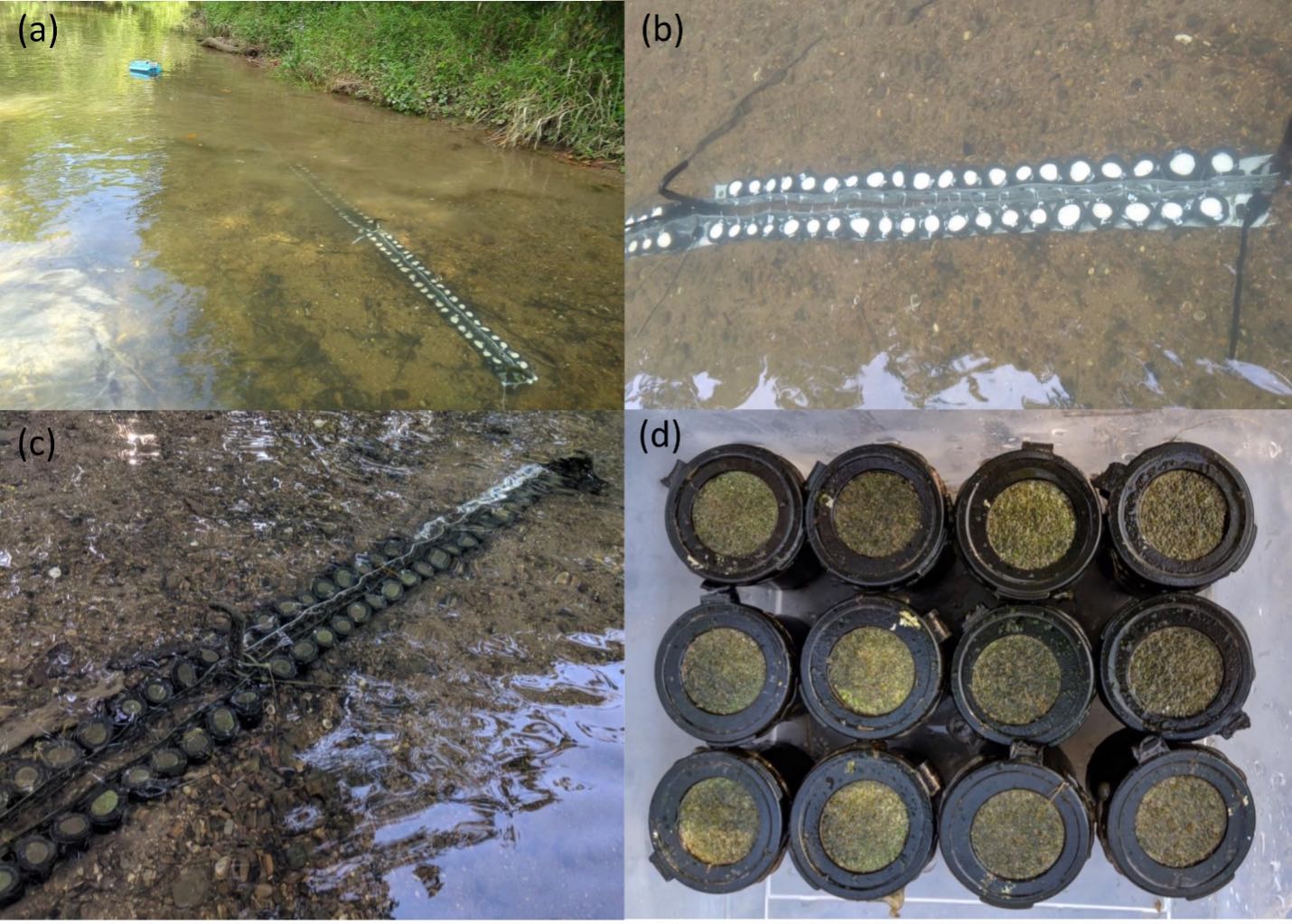
Researchers deployed nutrient diffusing substrates (a and b) in East Fork Poplar Creek in Oak Ridge, Tenn. Periphyton were allowed to colonize (c), and disks (d) were returned to the laboratory for community characterization and measures of mercury methylation.
[Reprinted under a Creative Commons Attribution 4.0 International License (CC BY 4.0) from Carrell, A., et al. "Nutrient Exposure Alters Microbial Composition, Structure, and Mercury Methylating Activity in Periphyton in a Contaminated Watershed." Frontiers in Microbiology 12 (543), 647861 (2021). DOI:10.3389/fmicb.2021.647861.]
The Science
The conversion of mercury (Hg) to monomethylmercury (MMHg) is a critical area of concern in global Hg cycling. Once MMHg is produced within an ecosystem, it is bioaccumulated in organisms and biomagnifies through food webs, ultimately impacting humans through the consumption of fish with elevated levels of MMHg. Understanding how MMHg is produced and stored in aquatic ecosystems is imperative for managing this pollutant. Aquatic microbial mats (periphyton) may produce significant amounts of MMHg, but little is known about the organisms that inhabit these mats or their role in Hg cycling. To improve this understanding, a research team from Oak Ridge National Laboratory characterized the structure and function of archaea, bacteria, fungi, and Hg-methylating microorganisms in periphyton grown in an East Tennessee contaminated watershed. They examined how nutrient amendments (nitrate and/or phosphate) altered periphyton community structure and function. Surprisingly, the team found that Hg methylation potential correlated with numerous bacterial families that do not contain hgcAB, the pair of genes responsible for Hg methylation. Furthermore, the nitrate treatment resulted in the most connected periphyton communities, which also had enhanced Hg-methylation potential. Results suggest that the overall structure of periphyton communities influences rates of Hg transformation within these microbial mats.
The Impact
This work provides insight into community interactions within the periphyton microbiome that may contribute to Hg cycling. Results suggest that monitoring the entire microbiome in addition to the Hg-methylating community will be important in evaluating Hg methylation-demethylation dynamics in natural environments. The study’s findings will inform future research focusing on establishing mixed microbial consortia to uncover mechanisms that drive shifts in Hg cycling within periphyton habitats.
Summary
The conversion of Hg to the contaminant MMHg is a critical concern in global Hg cycling. To better understand the Hg-methylating potential of the periphyton microbiome, researchers used high-throughput amplicon sequencing of the 16S rRNA gene, ITS2 region, and Hg-methylation gene pair (hgcAB) to characterize periphyton communities in a contaminated watershed. They examined how nutrient amendments (nitrate and/or phosphate) altered periphyton community structure and function. Researchers found that bacterial and archaeal richness decreased in summer and increased in autumn, while fungal diversity generally increased in summer and decreased in autumn. Interestingly, the Hg-methylating communities were dominated by Proteobacteria followed by Candidatus Atribacteria across both seasons. Surprisingly, Hg methylation potential correlated with numerous bacterial families that do not contain hgcAB, suggesting that the overall microbiome structure of periphyton communities influence rates of Hg transformation within these microbial mats. To further explore these complex community interactions, the research team performed a microbial network analysis and found that the nitrate treatment resulted in the highest number of hub taxa that also corresponded with enhanced Hg-methylation potential. This work will inform future research examining the drivers of Hg cycling within periphyton communities.
Principal Investigator
Scott Brooks
Oak Ridge National Laboratory
[email protected]
Funding
This research was sponsored by the Office of Biological and Environmental Research within the U.S. Department of Energy (DOE) Office of Science as part of the Critical Interfaces Science Focus Area at Oak Ridge National Laboratory, which is managed by UT-Battelle, LLC, for the DOE under contract DE-AC05-00OR22725.
References
Carrell, A., et al. "Nutrient Exposure Alters Microbial Composition, Structure, and Mercury Methylating Activity in Periphyton in a Contaminated Watershed." Frontiers in Microbiology 12 (543), 647861 (2021). https://doi.org/10.3389/fmicb.2021.647861.